Expo
ver canal
ver canal
ver canal
ver canal
ver canal
ver canal
ver canal
ver canal
Química Clínica
HematologíaInmunologíaMicrobiologíaPatologíaTecnologíaIndustria
Eventos
Webinars

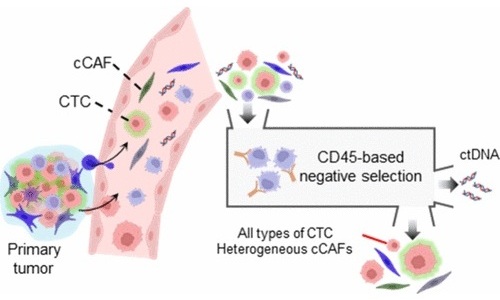
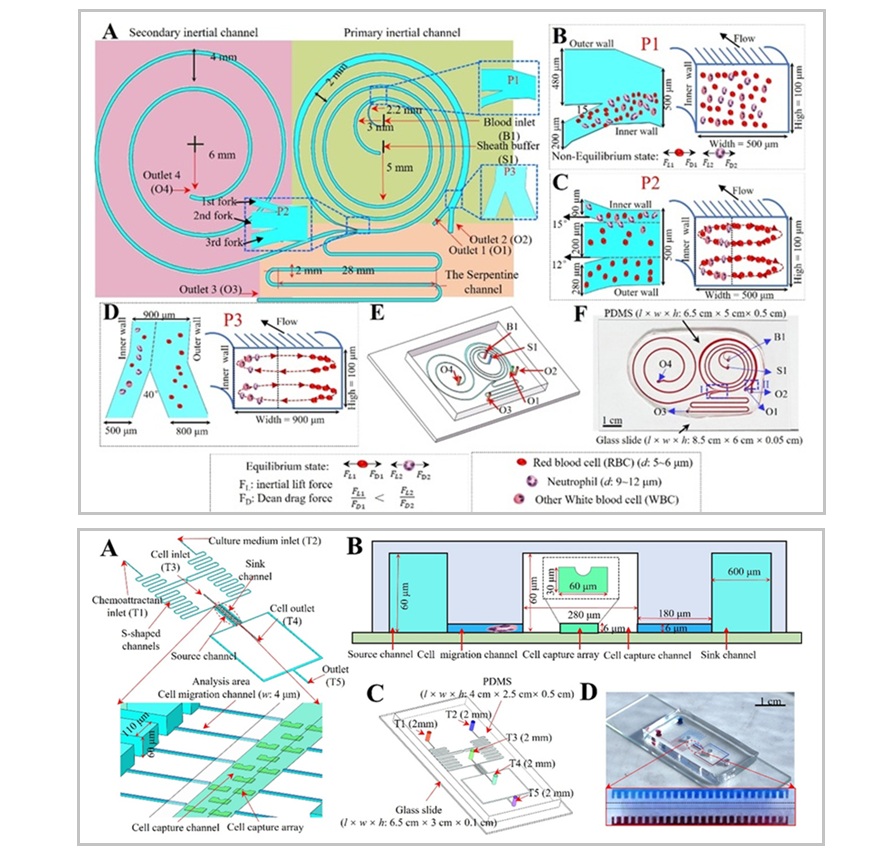
- Tecnología de aislamiento celular simultáneo mejora precisión del diagnóstico del cáncer
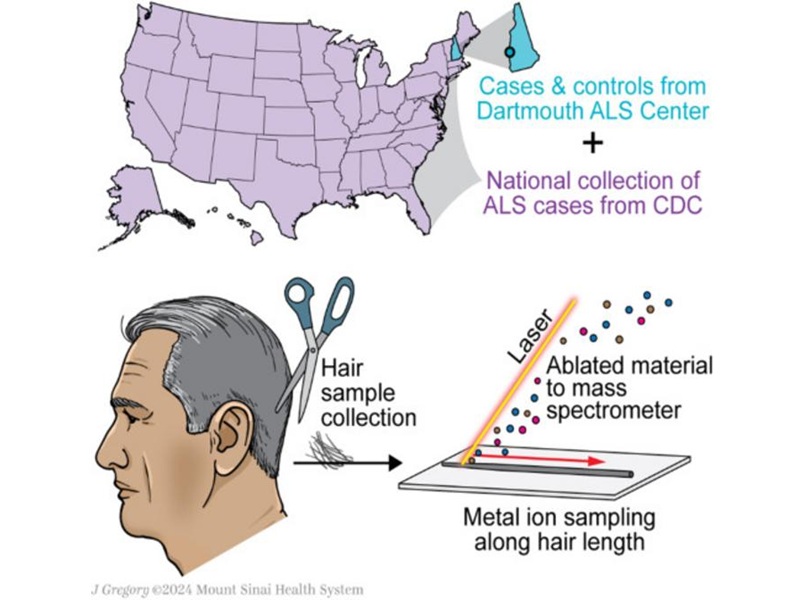
- Sencilla prueba capilar no invasiva podría acelerar diagnóstico de ELA
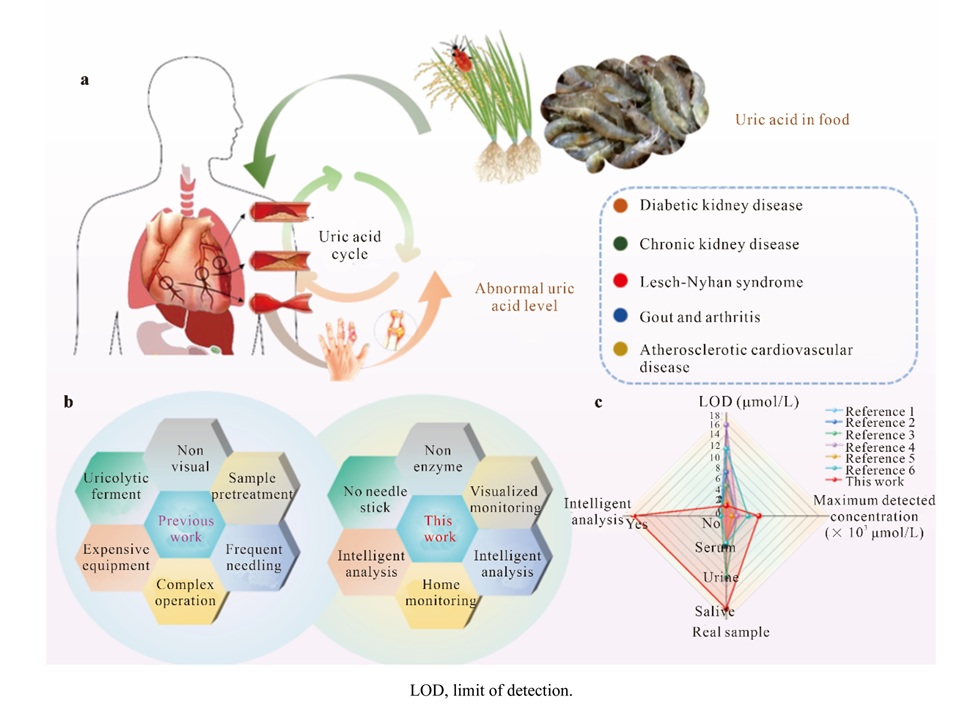
- Prueba de saliva detecta niveles elevados de ácido úrico sin necesidad de extraer sangre
- Marcadores de cáncer de próstata basados en composición química de calcificaciones aceleran detección
- Prueba de aliento ayuda a detectar cánceres de sangre
- Método basado en metilación del ADN predice progresión del cáncer
- Prueba de orina podría predecir resultado en trasplante de cartílago
- Análisis sanguíneo de cáncer en 2 horas transforma detección de tumores
- Prueba ultrasensible podría identificar primeros signos moleculares de recaída metastásica en pacientes con cáncer de mama
- Prueba de inmunoensayo automatizada de alto rendimiento impulsa investigación clínica neurodegenerativa
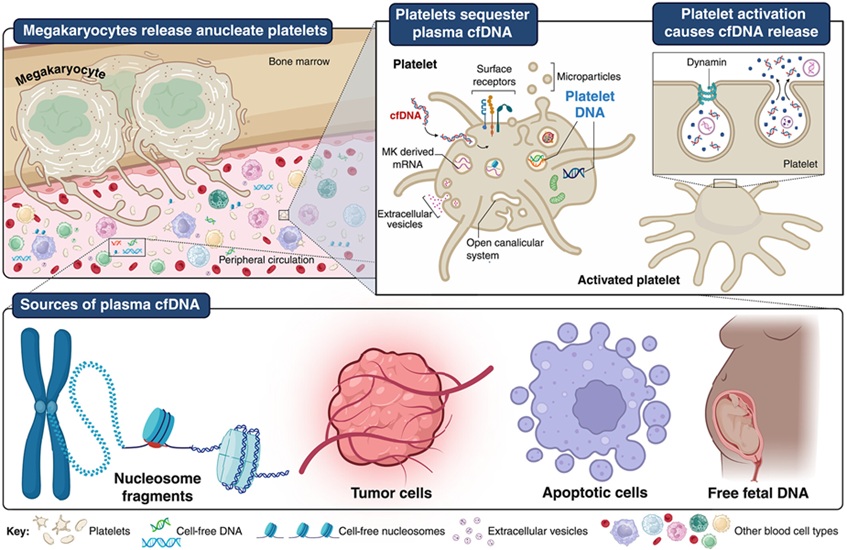
- Las plaquetas podrían mejorar detección temprana y mínimamente invasiva del cáncer
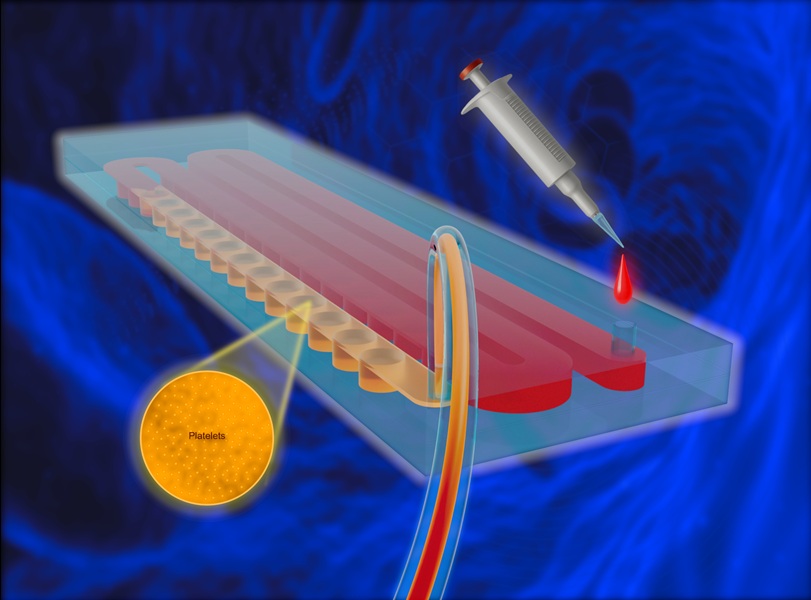
- Dispositivo portátil desechable obtiene plasma rico en plaquetas sin equipos complejos
- Prueba de cartucho desechable ofrece resultados de hemograma rápidos y precisos
- Primera prueba de monitorización de heparina POC proporciona resultados rápidos
- Nuevo sistema de puntuación predice riesgo de cáncer a partir de un trastorno sanguíneo común
- Nueva herramienta utiliza aprendizaje profundo para terapia de precisión contra cáncer
- Prueba diagnóstica complementaria identifica cáncer de mama y de vías biliares con HER2 ultrabajo
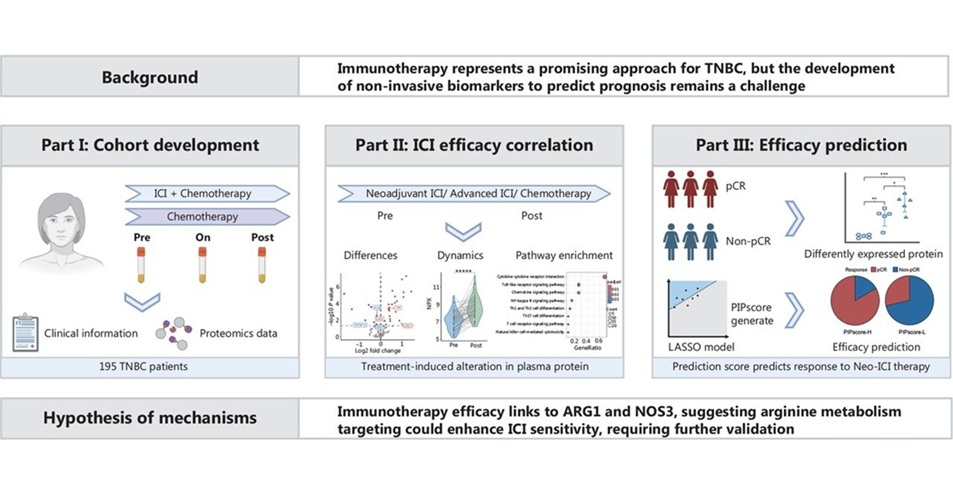
- Análisis sanguíneo predice eficacia de inmunoterapia en cáncer mamario triple negativo
- Pruebas genéticas simples podrían predecir éxito del tratamiento de esclerosis múltiple
- Nueva firma genética predice respuesta a inmunoterapia en cánceres renales avanzados
- Nuevo método diagnóstico confirma sepsis de forma más temprana
- Nuevos marcadores podrían predecir riesgo de infección grave por clamidia
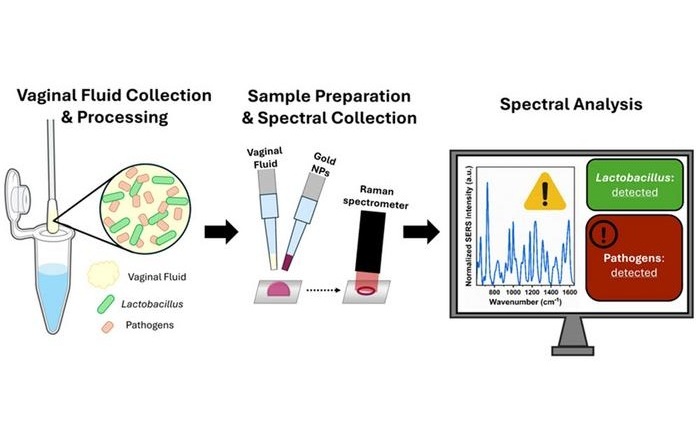
- Espectroscopia portátil detecta de forma rápida y no invasiva bacterias en fluido vaginal
- Prueba de saliva basada en CRISPR detecta tuberculosis en esputo
- Análisis de orina diagnostica infección pulmonar común en personas inmunodeprimidas
- Tecnología de diagnóstico rápido detecta infecciones del tracto respiratorio inferior en muestras de aliento
- Sensor de grafeno utiliza muestra de aliento para identificar diabetes y prediabetes en minutos
- Parche de sudor inalámbrico podría usarse como prueba diagnóstica para fibrosis quística
- Nuevo método mejora fiabilidad de la IA con aplicaciones en diagnóstico médico
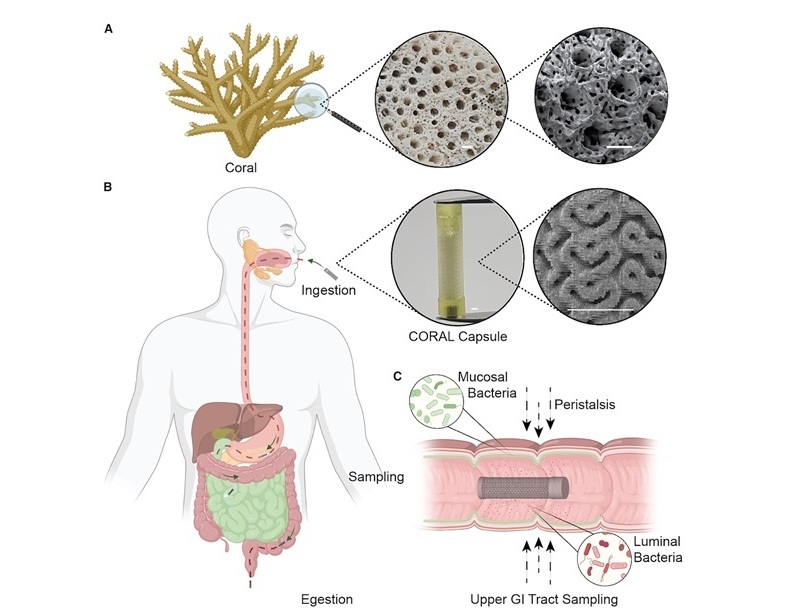
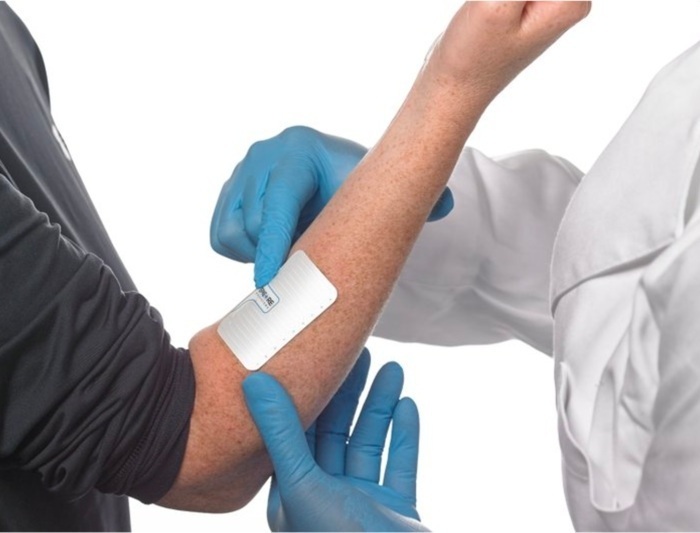
- Parche de microagujas autoalimentado recolecta muestras de biomarcadores sin extraer sangre
- Werfen y VolitionRx se asocian para adelantar pruebas diagnósticas del síndrome antifosfolípido
- Siemens Healthineers y Carna Health se asocian para impulsar innovación en cuidado renal
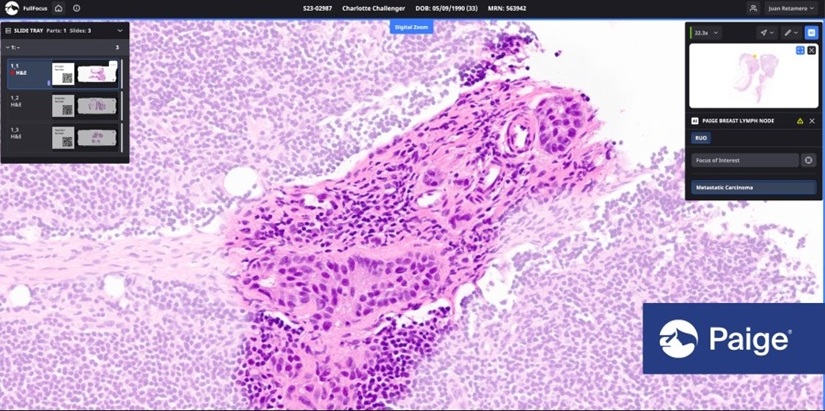
- Tempus AI adquiere empresa de patología digital Paige
- Vircell lanza nuevo sitio web para sus productos de control molecular
- Nuevo manual ayuda a médicos a diagnosticar y tratar mejor enfermedades crónicas asociadas a infecciones
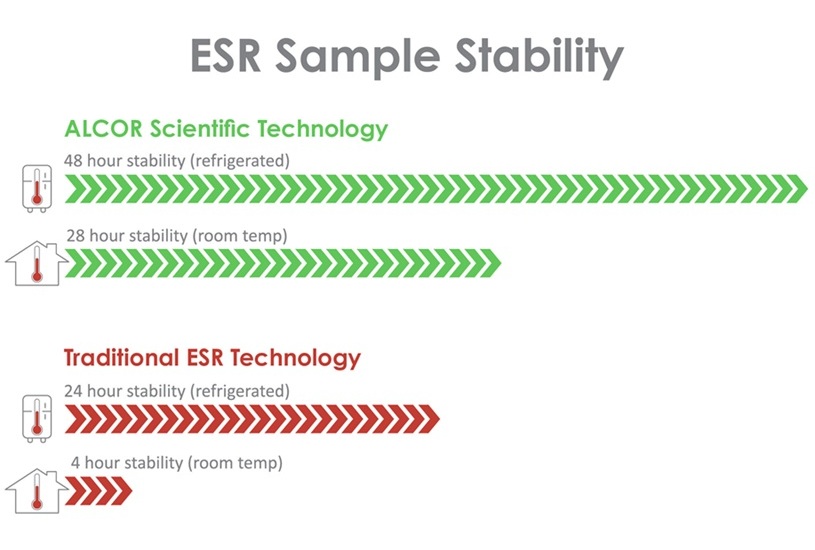
- Adelanto en VSG amplía estabilidad de muestras sanguíneas de 4 a 28 horas
- Análisis patológico preciso mejora resultados del tratamiento del fibrosarcoma en adultos
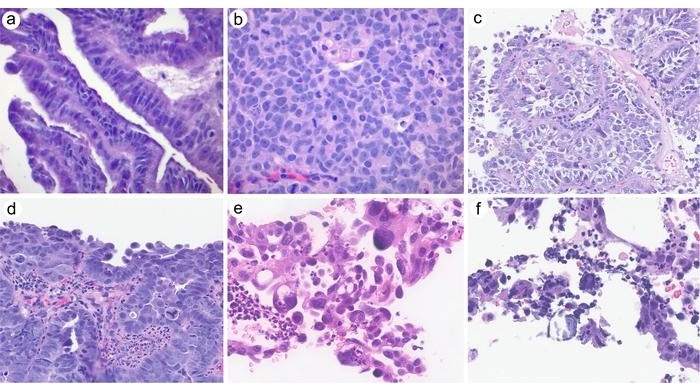
- Estudio clinicopatológico respalda exclusión del carcinoma seroso cervical de clasificación de OMS
- Sistema con IA compatible con dispositivos móviles revolucionará diagnóstico de malaria
- Microscopio compacto con IA permite puntuación del cáncer rápida y rentable

 Expo
Expo
- Tecnología de aislamiento celular simultáneo mejora precisión del diagnóstico del cáncer
- Sencilla prueba capilar no invasiva podría acelerar diagnóstico de ELA
- Prueba de saliva detecta niveles elevados de ácido úrico sin necesidad de extraer sangre
- Marcadores de cáncer de próstata basados en composición química de calcificaciones aceleran detección
- Prueba de aliento ayuda a detectar cánceres de sangre
- Método basado en metilación del ADN predice progresión del cáncer
- Prueba de orina podría predecir resultado en trasplante de cartílago
- Análisis sanguíneo de cáncer en 2 horas transforma detección de tumores
- Prueba ultrasensible podría identificar primeros signos moleculares de recaída metastásica en pacientes con cáncer de mama
- Prueba de inmunoensayo automatizada de alto rendimiento impulsa investigación clínica neurodegenerativa
- Las plaquetas podrían mejorar detección temprana y mínimamente invasiva del cáncer
- Dispositivo portátil desechable obtiene plasma rico en plaquetas sin equipos complejos
- Prueba de cartucho desechable ofrece resultados de hemograma rápidos y precisos
- Primera prueba de monitorización de heparina POC proporciona resultados rápidos
- Nuevo sistema de puntuación predice riesgo de cáncer a partir de un trastorno sanguíneo común
- Nueva herramienta utiliza aprendizaje profundo para terapia de precisión contra cáncer
- Prueba diagnóstica complementaria identifica cáncer de mama y de vías biliares con HER2 ultrabajo
- Análisis sanguíneo predice eficacia de inmunoterapia en cáncer mamario triple negativo
- Pruebas genéticas simples podrían predecir éxito del tratamiento de esclerosis múltiple
- Nueva firma genética predice respuesta a inmunoterapia en cánceres renales avanzados
- Nuevo método diagnóstico confirma sepsis de forma más temprana
- Nuevos marcadores podrían predecir riesgo de infección grave por clamidia
- Espectroscopia portátil detecta de forma rápida y no invasiva bacterias en fluido vaginal
- Prueba de saliva basada en CRISPR detecta tuberculosis en esputo
- Análisis de orina diagnostica infección pulmonar común en personas inmunodeprimidas
- Tecnología de diagnóstico rápido detecta infecciones del tracto respiratorio inferior en muestras de aliento
- Sensor de grafeno utiliza muestra de aliento para identificar diabetes y prediabetes en minutos
- Parche de sudor inalámbrico podría usarse como prueba diagnóstica para fibrosis quística
- Nuevo método mejora fiabilidad de la IA con aplicaciones en diagnóstico médico
- Parche de microagujas autoalimentado recolecta muestras de biomarcadores sin extraer sangre
- Werfen y VolitionRx se asocian para adelantar pruebas diagnósticas del síndrome antifosfolípido
- Siemens Healthineers y Carna Health se asocian para impulsar innovación en cuidado renal
- Tempus AI adquiere empresa de patología digital Paige
- Vircell lanza nuevo sitio web para sus productos de control molecular
- Nuevo manual ayuda a médicos a diagnosticar y tratar mejor enfermedades crónicas asociadas a infecciones
- Encuentran etiologías de COVID prolongada en muestras de sangre con infección aguda
- Dispositivo novedoso detecta anticuerpos contra la COVID-19 en cinco minutos
- Prueba para COVID-19 mediante CRISPR detecta SARS-CoV-2 en 30 minutos usando tijeras genéticas
- Asocian disbiosis del microbioma intestinal con la COVID-19
- Validan prueba rápida novedosa de antígeno para el SARS-CoV-2 con respecto a su exactitud diagnóstica
- Adelanto en VSG amplía estabilidad de muestras sanguíneas de 4 a 28 horas
- Análisis patológico preciso mejora resultados del tratamiento del fibrosarcoma en adultos
- Estudio clinicopatológico respalda exclusión del carcinoma seroso cervical de clasificación de OMS
- Sistema con IA compatible con dispositivos móviles revolucionará diagnóstico de malaria
- Microscopio compacto con IA permite puntuación del cáncer rápida y rentable